Wuhan Leader Medical Biotechnology Co., Ltd. is an R&D and manufacturing platform for biomedical materials that combines production and research, allowing young talents to achieve their flying dream. It’s mainly engaged in the R&D, production, and sales of Class III implanted biomedical materials and tissue regeneration materials. The initiator of the company returns from abroad study, also a chief physician and the founder of the Osteolink Biomaterial Co., LTD, a company with a 20-year history of R&D and production of biomedical implanted materials. Our vision is to develop cutting-edge medical devices that integrate advanced technology, ensuring reliable performance and user-friendliness. The research and development fields are artificial bone materials, biological bone implant materials, biomembrane repair materials, collagen membranes, and orthopedic hemostatic materials.






The company’s headquarters is located in the National Medical Instrument Park of the East Lake High-Tech Zone in Wuhan. For upgrading research and innovation, with a vision to develop a worldwide market, we also established Hong Kong Leader Biomaterial Co., Ltd and built an advanced R&D lab in the world’s cutting-edge R&D platform, Hong Kong Science Technology Park(HKSTP). The headquarters have more than 16,000 square meters of buildings, for office work, R&D, and production. Our advanced laboratory includes 10 thousand level cleanrooms for Pilot tests, R&D and production, a clean inspection room, a physical and chemical laboratory, a primary processing laboratory, a warehouse for raw materials, semi-finished products, and fully-finished products. We are also equipped with more than hundreds of high-end laboratory equipment, such as purified water, freeze-drying, high-speed refrigerated centrifuge, biological safety cabinet, stability test chamber, stability test chamber, and reactors.
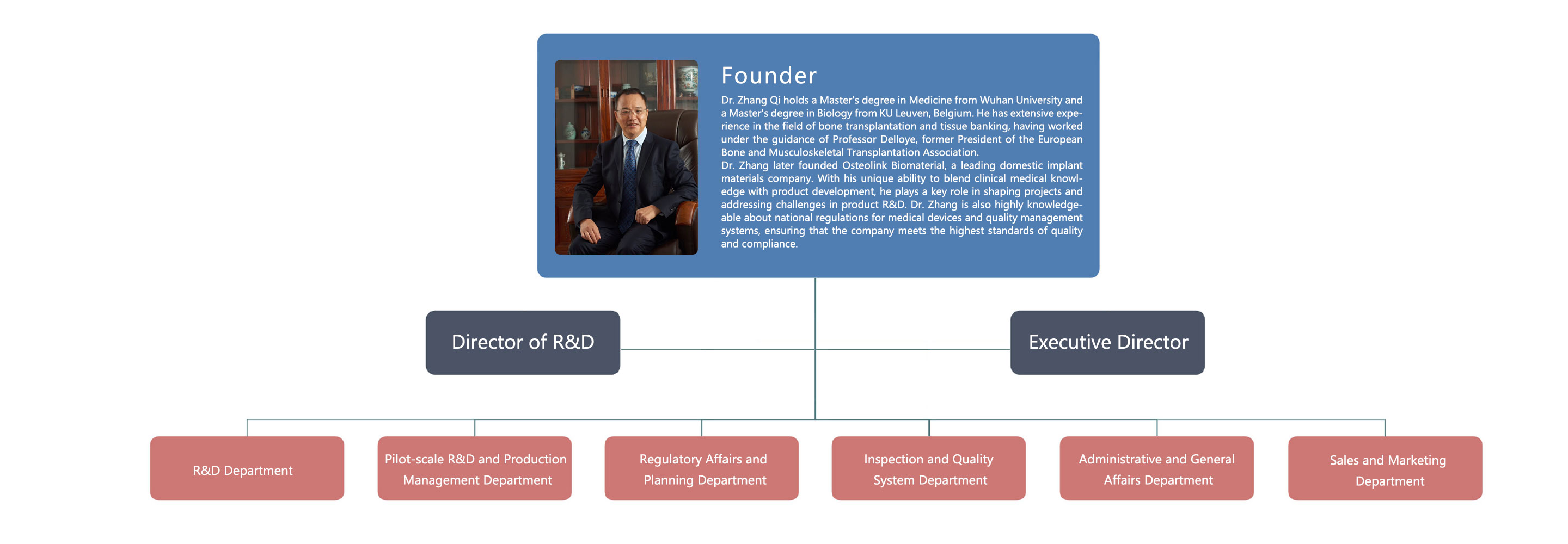
The company team consists of clinical doctors, chemical and biomaterials engineers, registered regulatory and planning talents, and quality control and quality system experts. Having complete capabilities and experience in research and development, testing, quality management systems, registration management, and production of biological implant materials for medical devices.
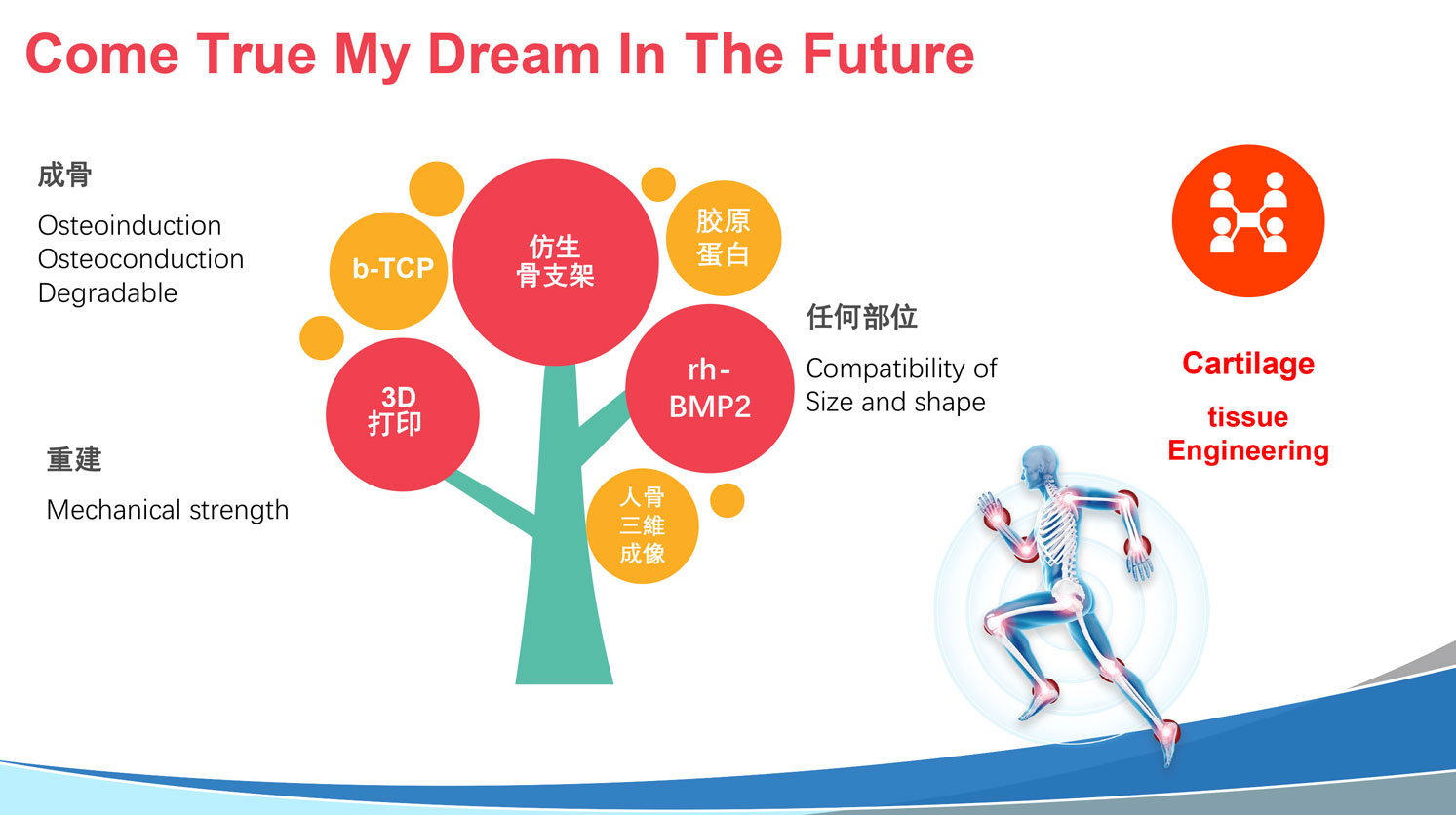
Upholding the principles of innovation and quality as equal priorities, we strive to deliver products that enhance both efficiency and safety in the medical field. Through continuous technological breakthroughs and rigorous quality control, we ensure that every product provides maximum value and convenience to both physicians and patients in real-world clinical settings. Our ultimate goal is to drive sustained progress in the healthcare industry by offering exceptional medical devices that contribute to better treatment outcomes and improved quality of care.

| Recruitment Position | Number of Recruitments | Department | Work Location | Release Date | Job Details |
|---|---|---|---|---|---|
| 医疗器械工程师 | 2人 | 研发部 | 武汉 | 2025-02-27 | View Detail |
| 质检员 | 3人 | 检验与质量体系部 | 武汉 | 2025-02-25 | View Detail |
| 生产操作员 | 3人 | 中试研发与生产管理部 | 武汉 | 2025-02-20 | View Detail |
| 设备管理员 | 2人 | 中试研发与生产管理部 | 武汉 | 2025-02-19 | View Detail |
| 销售 | 3人 | 销售与市场部 | 不限 | 2025-02-18 | View Detail |