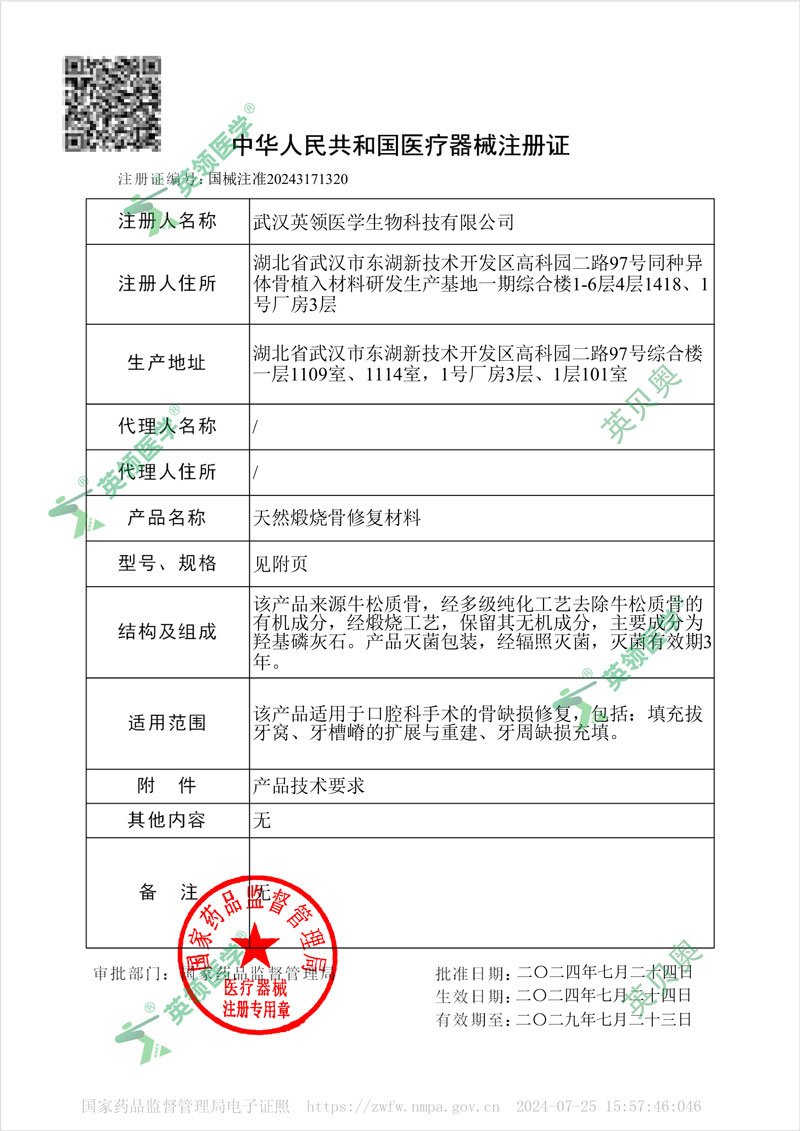
On July 24, 2024, Wuhan Leader Med-Biotech Co., Ltd. successfully obtained the Chinese Medical Device Registration Certificate (Registration No. 20243171320) for its independently developed Natural Calcined Bone Graft Material. This approval marks another significant milestone in our journey within the healthcare sector.
This certificate is not only a strong endorsement of the product’s quality, safety, and efficacy, but also a recognition of our commitment to continuous innovation and improving medical services. It serves as a passport, opening doors to broader market opportunities and enabling more patients to benefit from our carefully developed medical solutions.