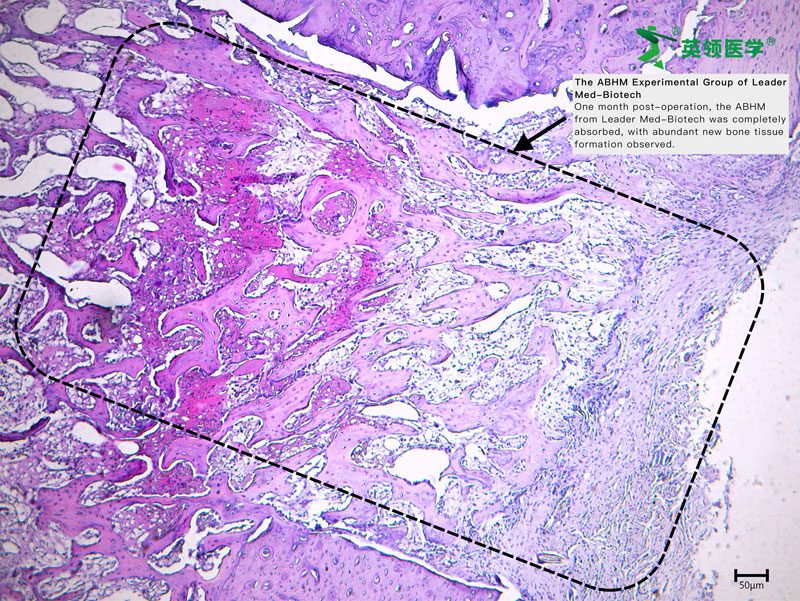
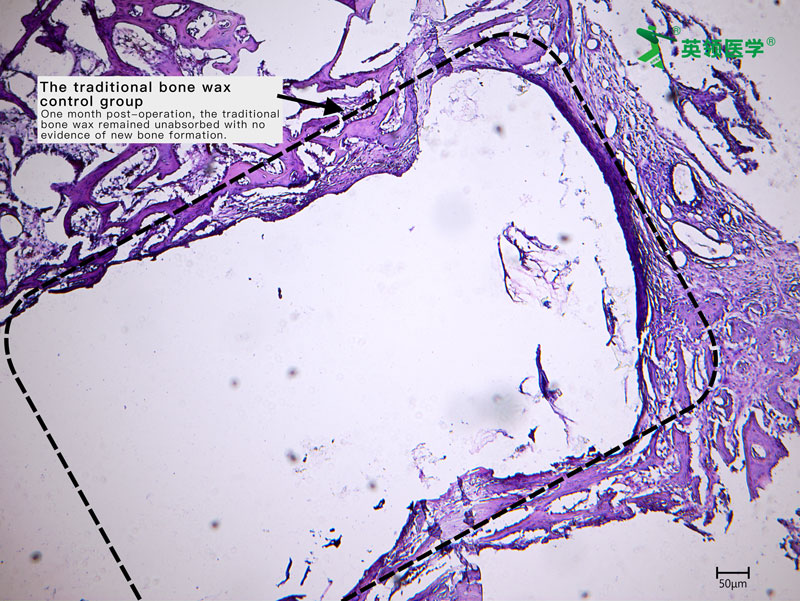
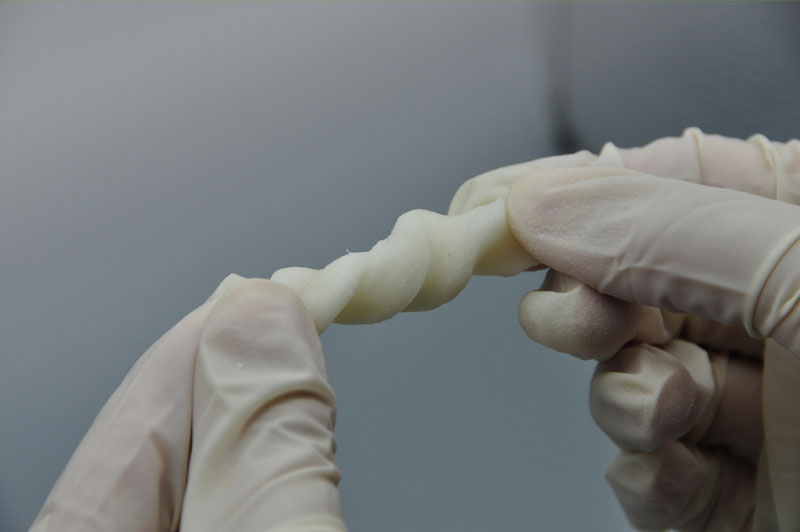
The multicenter clinical trial for Absorbable Bone Wound Hemostasis Mud (ABHM), jointly developed by our company and Zhongnan Hospital of Wuhan University, has recently been successfully completed. As of last week, a total of 218 clinical cases had been performed across participating clinical centers, with full data collection and follow-up for all cases expected to be finalized by next week.
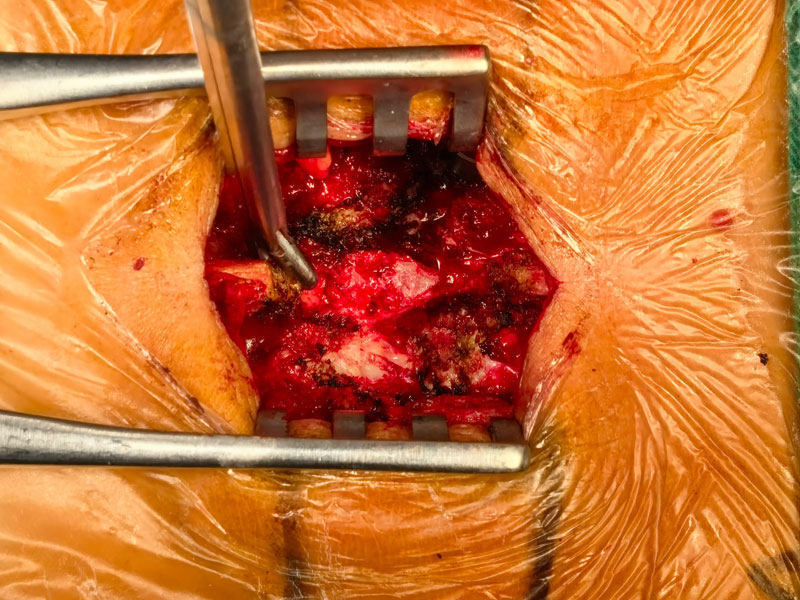
During the trial, ABHM demonstrated excellent clinical performance and safety. Its biodegradable properties and ease of use were highly praised by surgical experts from multiple clinical centers. The product rapidly achieves hemostasis by mechanically sealing bone wound surfaces, and it degrades completely within four weeks. This effectively avoids complications associated with traditional bone wax, such as impaired bone healing, foreign body granuloma, chronic inflammation, and infection—offering a safer and more physiologically compatible solution for bone wound management.
The successful completion of this multicenter clinical trial marks a critical step toward the formal clinical application of ABHM and provides important support for advancing the innovation and development of high-performance absorbable hemostatic materials. Moving forward, we will continue to promote product registration and industrialization, committed to delivering superior bone wound management solutions for clinical practice.